The singlet-triplet (S1/T1) gap of an organic chromophore is a decisive property for various photophysical applications. There are well-established rules for minimizing S1/T1 gaps. Essentially all one has to do is separate the HOMO and LUMO in space and this minimises the HOMO/LUMO exchange integral and thus the S1/T1 gap.
Maximising S1/T1 gaps is a different story. Simply trying to maximise HOMO/LUMO overlaps does not help by itself help. And so far it was not clear what to do instead.
We investigated this question in a recent article:
W. Zeng, C. Zhong, H. Bronstein, F. Plasser
“Understanding and Tuning Singlet-Triplet (S1/T1) Energy Gaps in Planar Organic Chromophores”
Angew. Chem. Int. Ed., 2025, e202502485
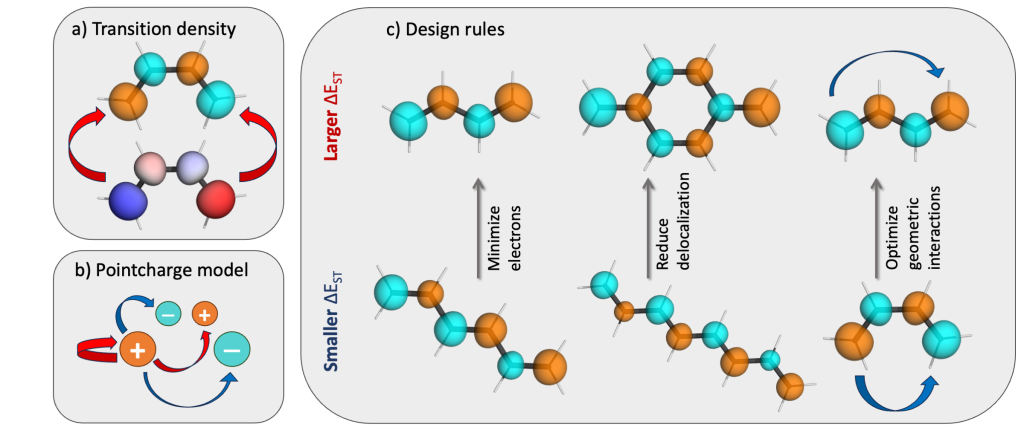
The developed strategy is summarised below. Starting from the realisation that the S1/T1 gap reflects the self-repulsion of the transition density [Phys. Chem. Chem. Phys., 2020, 22, 6058], we decompose this interaction via a formal pointcharge model. A large S1/T1 gap now corresponds to maximising repulsive interactions and minimising attractive interactions within this model. Doing so leads to three new rules for maximising S1/T1 gaps in planar organic chromophores:
- Minimising the number of π-electrons: smaller molecules generally have larger S1/T1 gaps.
- Reducing delocalisation: the S1/T1 gap goes up if they excitation can be localised on a subset of the carbon atoms.
- Optimising through-space geometric interactions: to maximise S1/T1 gaps one most avoid s-cis type 1,4-interactions.