The molecular orbital (MO) picture has become the standard tool used by chemists to understand molecular electronic structure and particularly excited states. In our newest paper Toward an understanding of electronic excitation energies beyond the molecular orbital picture, which just appeared as a Perspective in PCCP, we asked whether we can go beyond the MO picture in a systematic way in our understanding of excited states. This endeavour ended up more involved than initially expected but we hope that it presents some interesting physics in a memorable and sufficiently intuitive way.
Below, we exemplify the tools developed to study a donor-acceptor-donor system initially developed in JACS, 2014, 136, 18070 and revisited in PCCP, 2019, 21, 10580. The HOMO of this system is on the carbazole donor whereas the LUMO is on the anthraquinone acceptor. The lowest excited state of the system (in gas phase) is not reached via the HOMO-LUMO transition but it is a locally excited state on anthraquinone. Why?
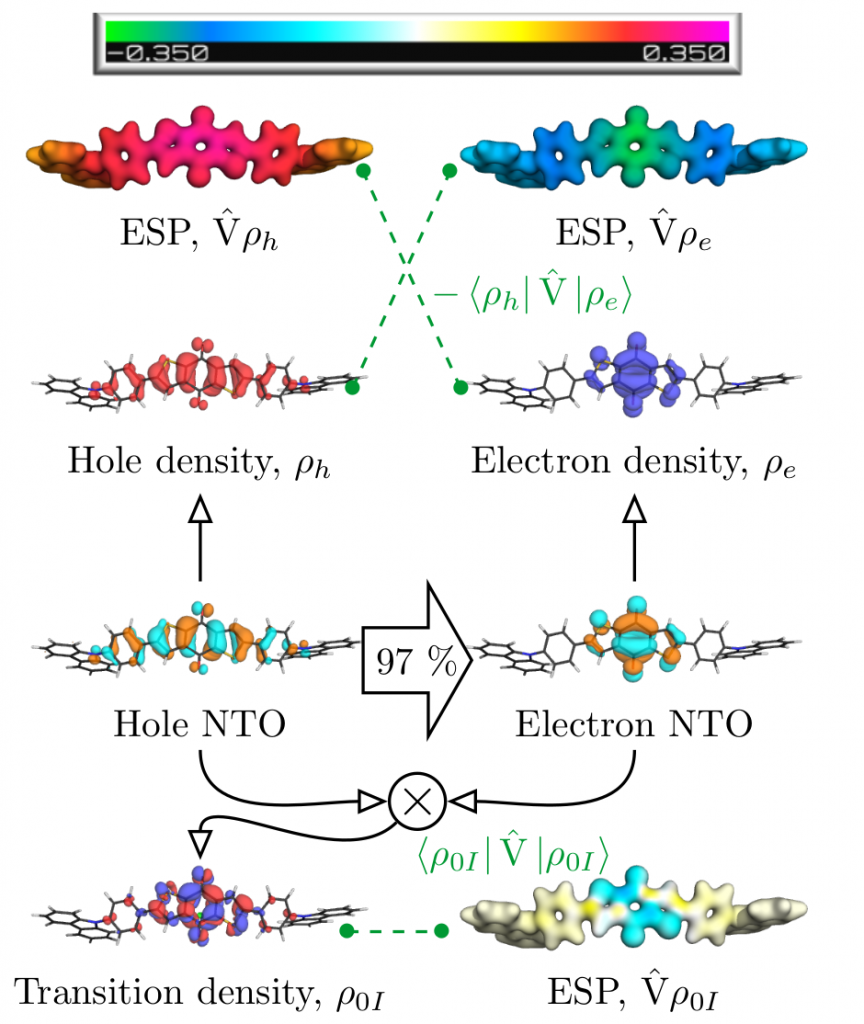
The contributions affecting the energy of the first excited singlet state are shown in Figure 1, above. In the third row the natural transition orbitals (NTOs) are shown. These give rise to the density of the excitation hole (red) and the excited electron (blue), shown above. The Coulomb attraction between these two charge distributions now stabilises the excited state. This Coulomb attraction is represented by the electrostatic potentials (ESPs) induced by the charge distributions. The important observation is that these ESPs are non-uniform with stronger contributions in the centre. Thus, the locally excited state is stabilised and an energetic penalty is paid for charge transfer. At the bottom of Figure 1, the transition density is shown, which leads to a repulsive exchange interaction destabilising local singlet states. In the present case the Coulomb attraction dominates and the lowest state is fairly local in nature.