A recent study led by F. Glöcklhofer from Imperial College, London, investigates the properties of substituted conjugated macrocycles. The first (pre-review) version just appeared on Open Research Europe:
[2.2.2.2]Paracyclophanetetraenes (PCTs): cyclic structural analogues of poly(p‑phenylene vinylene)s (PPVs) by M. Pletzer, F. Plasser, M. Rimmele, M. Heeney, and F. Glöcklhofer.
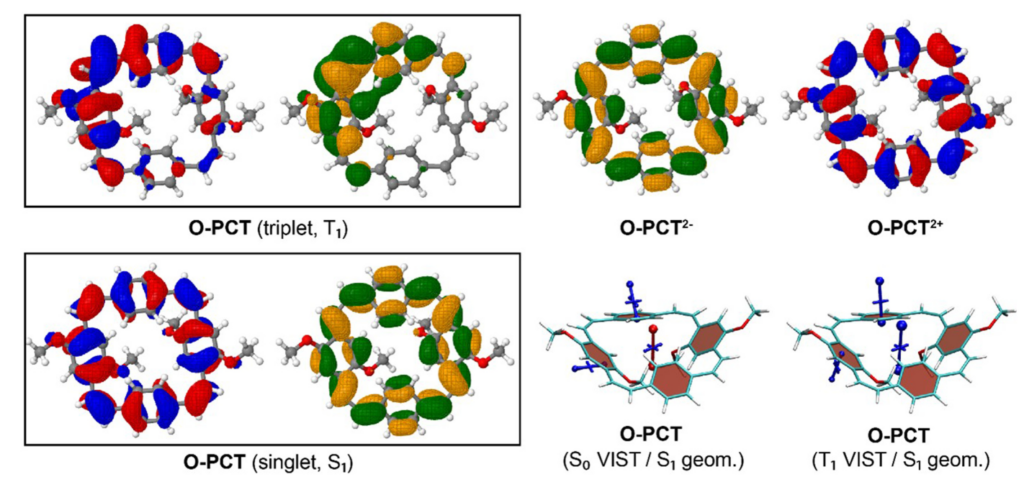
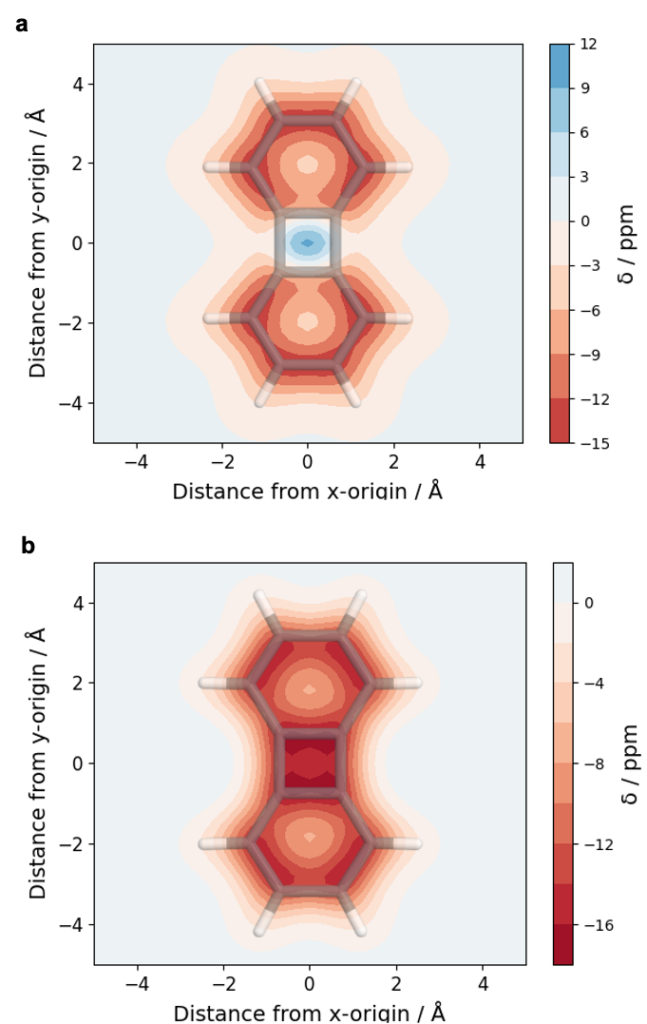
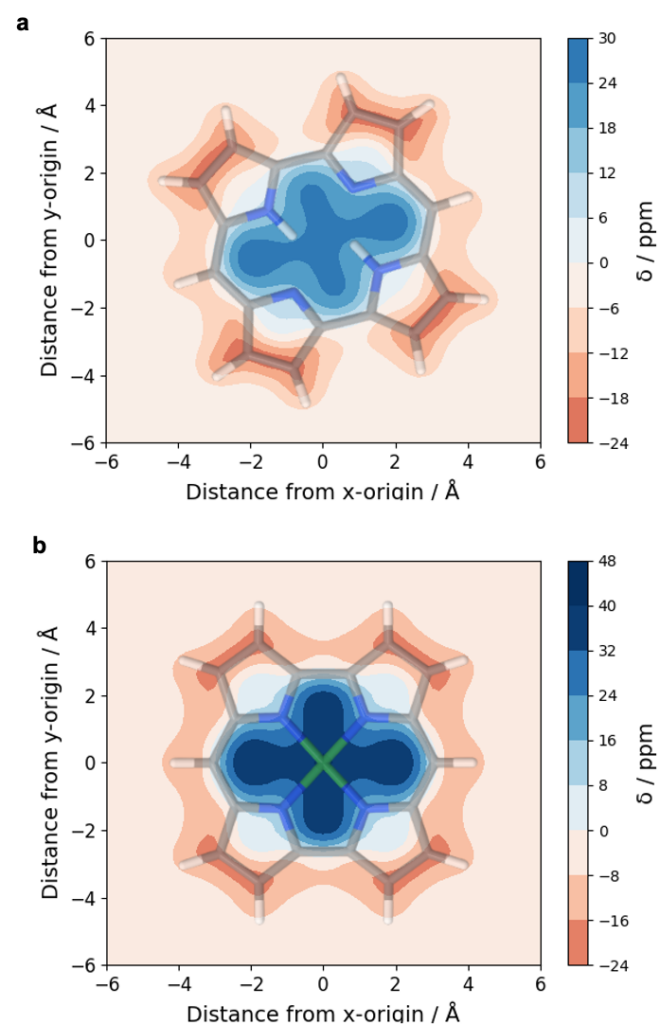
Computations reveal the local and global aromaticity of the macrocycles in various electronic states by using the VIST method along with a discussion of the nodal structures of the orbitals.