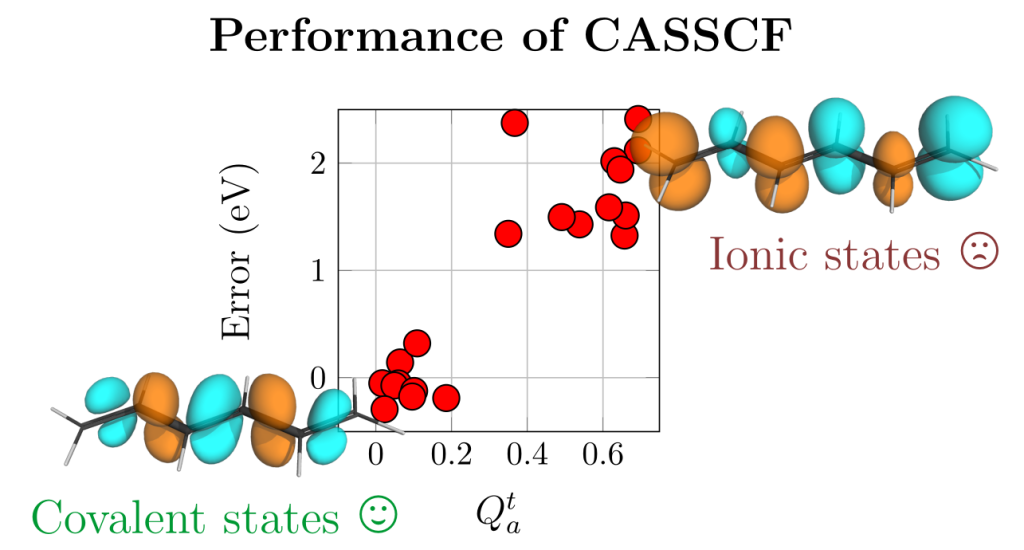
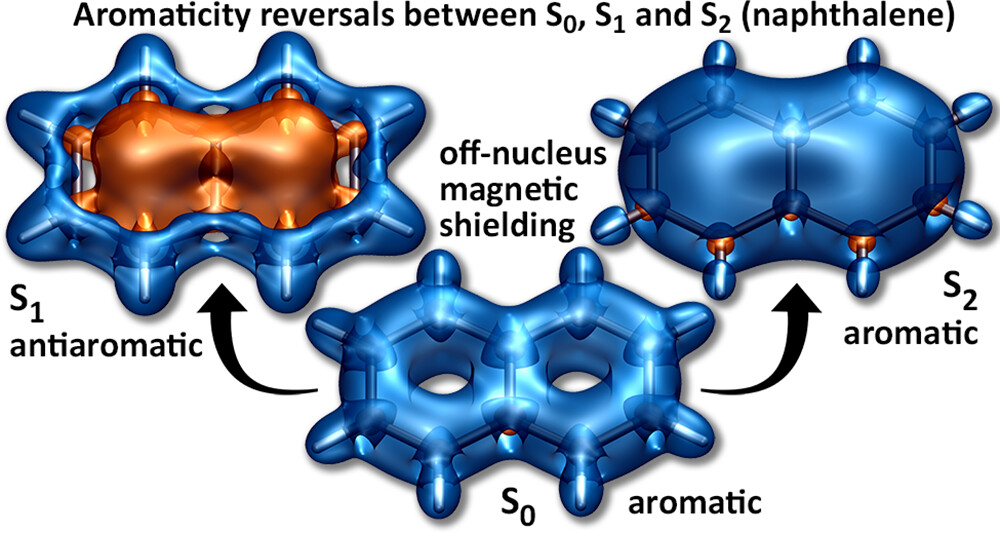
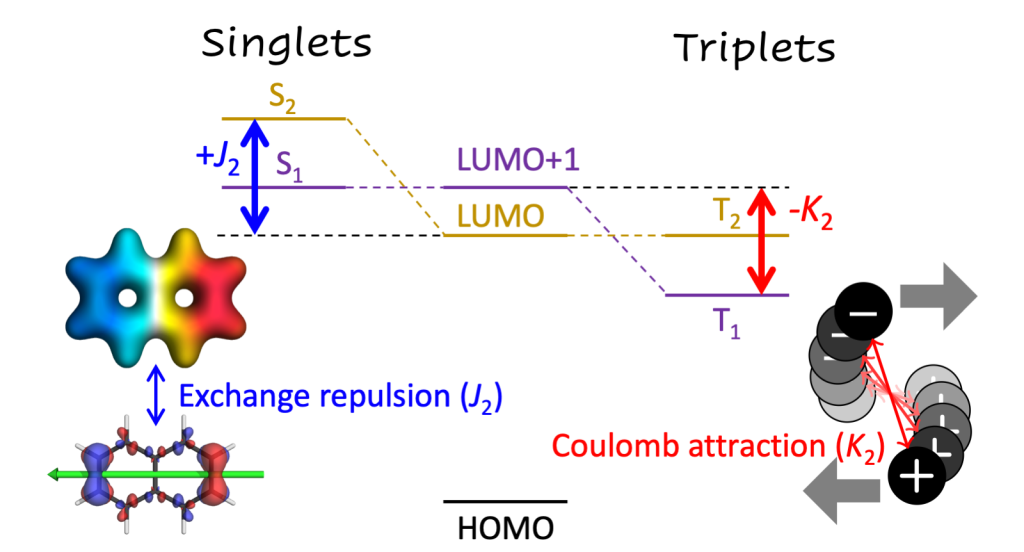
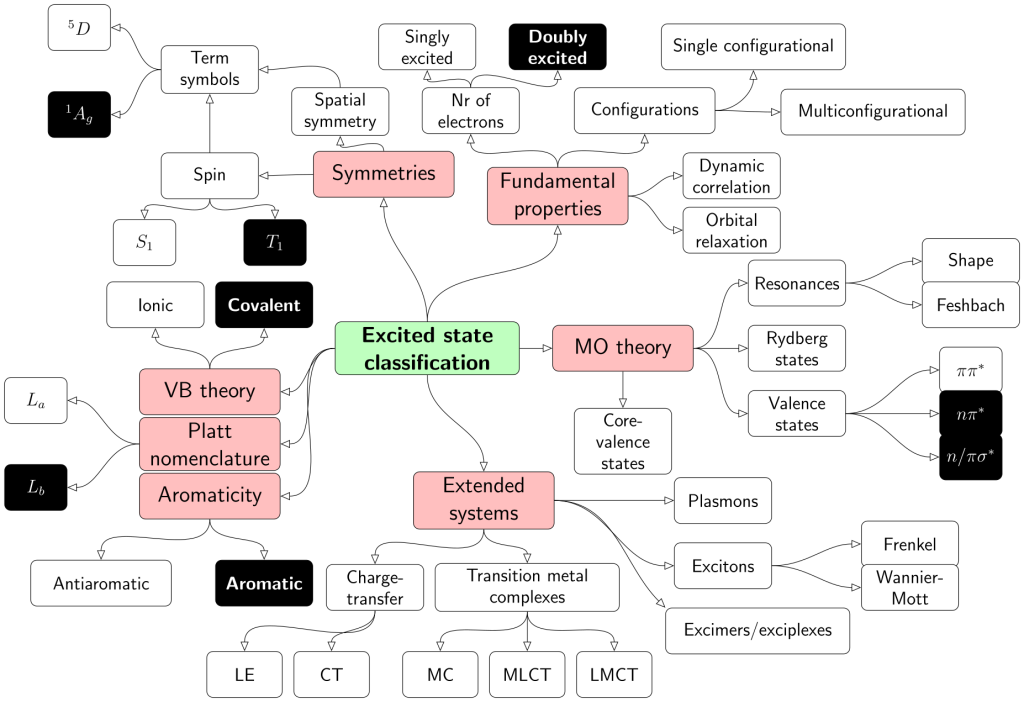
Applications are invited for a postdoctoral research associate position in computational chemistry at Loughborough University. This is a DSTL funded position to work with Dr Kenny Jolley and Dr Felix Plasser (Chemistry, Loughborough University) on predicting the crystal structure, dynamics and physical properties of energetic materials.
The successful applicant will conduct molecular dynamics (MD) and accelerated MD simulations on energetic compounds. Bulk physical properties, stability to shock and heat, and reaction pathways will be modelled and compared to experimental data. In a later stage, we will perform time-resolved simulations of explosion processes.
This position is ideally suited for an ambitious early career researcher with a background in computational chemistry and materials modelling. The successful candidate will be highly motivated with a strong research track record and a desire to pursue multidisciplinary research.
Feel free to contact me or Dr Kenny Jolley for informal equiries.
Closing date for applications is 24/11/2023, please follow this link for further info.