Today, Felix will present at the School on High-Performance Multilayer Molecular Dynamics Approaches in Madrid. He will talk about classification and analysis of excited states in the morning, and present the practical use of TheoDORE in the afternoon. Please, see the official web page for online joining instructions.
Author: Felix
Non-Kasha fluorescence
Kasha’s rule states that fluorescence generally occurs from the lowest excited singlet state (S1). Exceptions to this rule are usually associated with a metastable S2 state that is separated from S1 not allowing for interconversion. In a recent article we outlined a different mechanism for non-Kasha fluorescence: If S1 and S2 are very close in energy, then S2 is populated in a dynamic equilibrium following Boltzmann statistics. This effect is particularly pronounced if there is a large amount of vibrational excess energy following excitation into a high-energy absorption peak. The full story, “Non-Kasha fluorescence of pyrene emerges from a dynamic equilibrium between excited states” was just published in J. Chem. Phys.
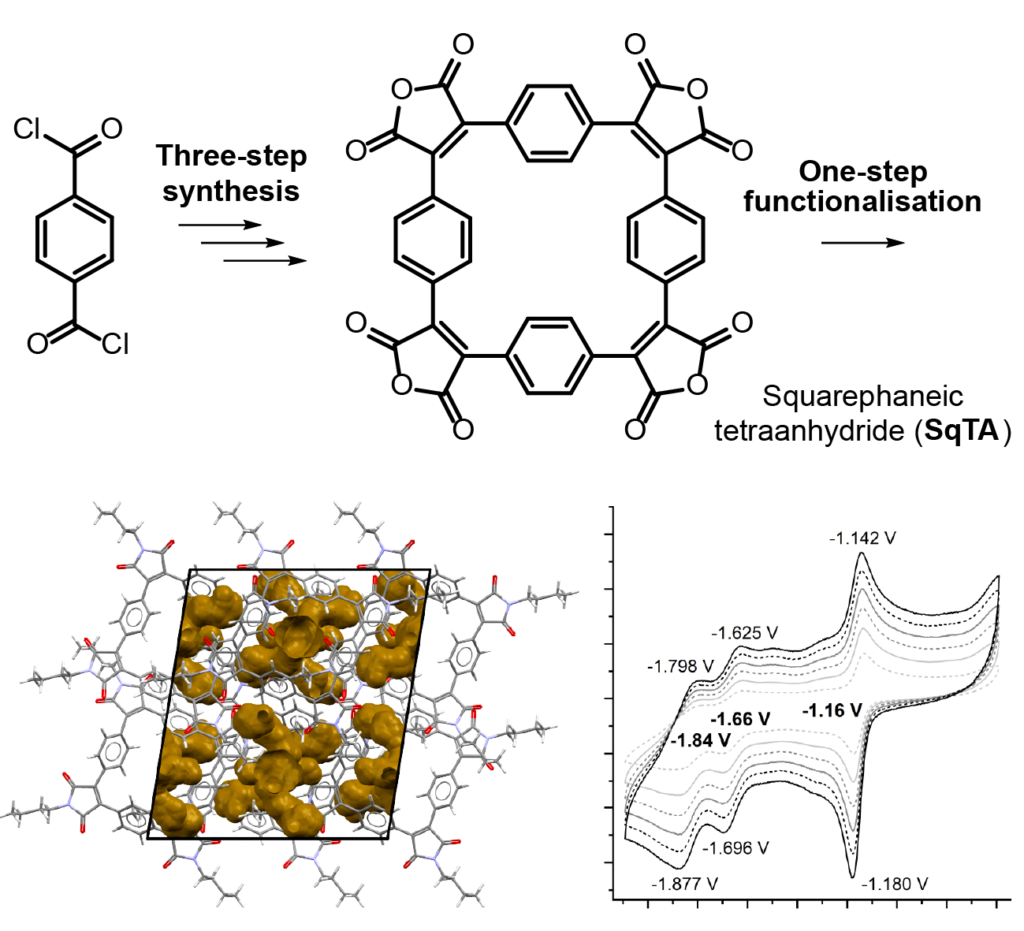
Squarephaneic Tetraanhydride
Our new paper “Squarephaneic Tetraanhydride: A Conjugated Square-Shaped Cyclophane for the Synthesis of Porous Organic Materials” was just published in Angewandte Chemie. This paper, led by Florian Glöcklhofer from Imperial college, explores the redox chemistry, porosity, and chemical derivatives of a newly developed tetraanhyride based on a macrocycle with a formally antiaromatic ground state.
Hydrogen-bonded Organic Framework
Our new paper “Hierarchical Assembly of a Micro-and Macroporous Hydrogen-Bonded Organic Framework with Tailored Single-Crystal Size,” led by Antonio Fernandez at Loughborough, just appeared in Angewandte Chemie.
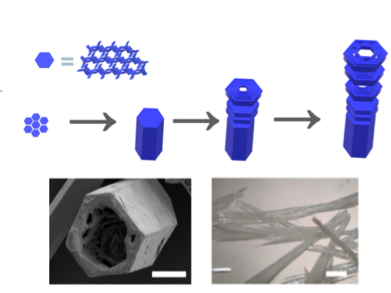
The work shows how a highly porous framework can be constructed by using different types of intermolecular interactions.
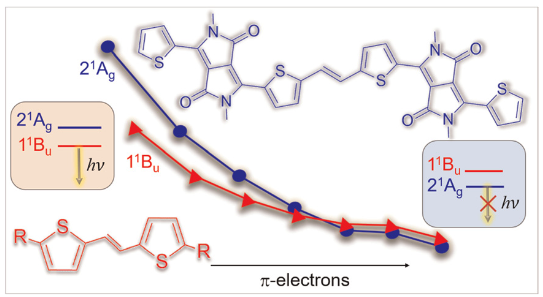
Diketopyrrolopyrroles
Our new paper Using diketopyrrolopyrroles to stabilize double excitation and control internal conversion, led by Mariana do Casal and Mario Barbatti from Aix Marseille University, just appeared in PCCP. This work highlights how double excitation character can support internal conversion. Wavefunction analysis using TheoDORE sheds light into the wavefunctions involved.
Eu(III) complexes
Our new paper “Impact of Varying the Phenylboronic Acid Position in Macrocyclic Eu(III) Complexes on the Recognition of Adenosine Monophosphate“, led by S. E. Bodman and S. J. Butler from Loughborough, just appeared in Organic Chemistry Frontiers. The paper is the second in a series studying the anion sensing properties of Eu(III) complexes with phenylboronic acids.
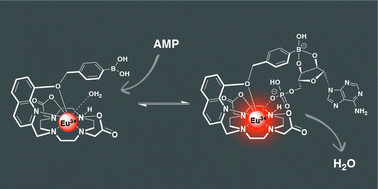
Aside from reporting the synthesis and anion binding, the paper presents new strategies for the computational analysis of such complexes. Aside from modelling the geometries by density functional theory, high-level multireference methods in OpenMolcas were applied to study the luminescence properties. These first principles computations offer a promising approach to access the emission spectra of lanthanide complexes, aiding the design of responsive lanthanide probes with specific photophysical properties
Magnetically induced currents
On the 14th of September Felix will give a talk at MAGIC 2022 (4th workshop on MAGnetically Induced Currents in molecules).
Title: “Bridging between the magnetic and molecular orbital pictures of excited-state aromaticity”
You can download the slides here.
Release of TheoDORE 3.0
Version 3.0 of the TheoDORE wavefunction analysis package is available. Download the current version below.
New features of TheoDORE 3.0
- New user interface and documentation
- Improvement for VIST (plot_vist)
- Improvements for natural orbital analysis (analyze_nos) including unrestricted orbitals
- LOC for ionic states (analyze_tden)
- Jmol densities (jmol_mos)
- State-to-state TDM
- Updated ADF interface
- ONETEP interface
- Excitation number, modified from [DOI: (10.1021/acs.jctc.7b00963)]
Note: TheoDORE 3 has a modified user interface. To use TheoDORE call
theodore theoinp
theodore analyze_tden
theodore analyze_nos
etc.
Download the newest release of the TheoDORE wavefunction analysis program – TheoDORE 3.2 (22 July 2024)

Full release notes
Continue readingCOLUMBUS open-source release
The COLUMBUS programme package – a collection of programs for high-level ab initio molecular electronic structure calculations – has been released open-source. Please find
- the new webpage,
- the code repository.
Any contributions (from small bugfixes to major features) are welcome. You can find more information about contributing here.
10th OpenMolcas Developers’ Workshop
Tomorrow, Felix will give a talk at the 10th OpenMolcas Develepors’ Workshop.
Content covered
- Applications of OpenMolcas to compute luminescence spectra of lanthanide complexes
- The Columbus/OpenMolcas interface and the new Columbus open-source release