Aromaticity is a ubiquitous yet elusive concept in chemistry and chemists have spent a great deal of effort on developing methods to quantify and visualise aromaticity. One particularly popular method is the nucleus independent shift (NICS), which can be seen as a virtual NMR experiment carried out within a conjugated ring to evaluate the enhanced chemical shielding induced by aromatic ring-currents. Strikingly NICS also allows to quantify antiaromaticity, as this induces a net deshielding effect within the ring. NICS provides a powerful quantitative aromaticity criterion but the main challenge for its graphical representation is that the chemical shielding is a 3×3 tensor, which is difficult to visualise with the existing methods.
Therefore, we have developed a new method for the visualisation of chemical shielding tensors (VIST), which provides a local representation of the shielding tensor along with the molecular structure. The method, thus, allows to probe local aromaticity along with the underlying anisotropy of the shielding. The method is described in the preprint “3D Visualisation of chemical shielding tensors to elucidate aromaticity and antiaromaticity” available on ChemRxiv.
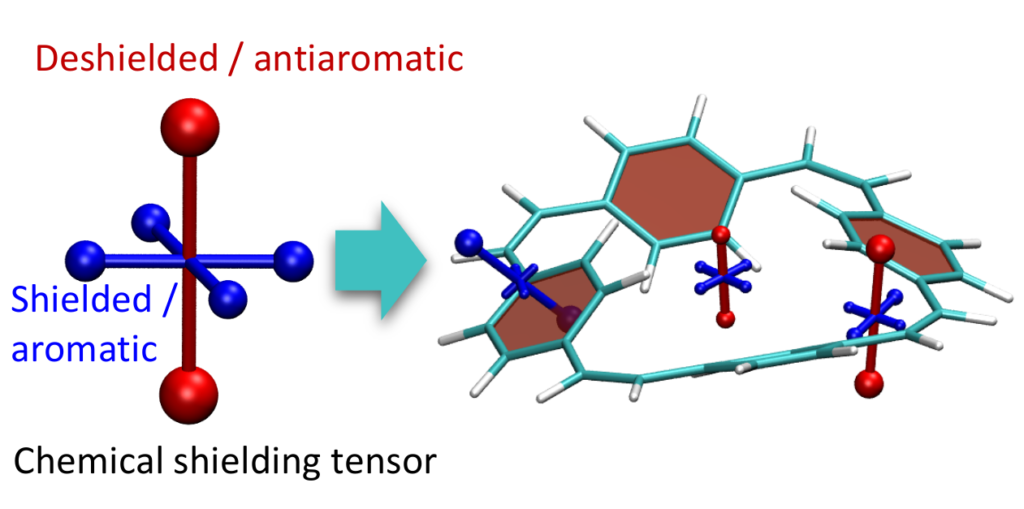
Within the preprent we exemplify the main concepts in the benzene and phenanthrene molecules and continue by studying
- the interplay of ground state antiaromaticity and Baird triplet state aromaticity in the potential singlet fission chromophore cyclobuta[l]phenanthrene,
- local aromaticity in the neutral formally antiaromatic ground state along with global aromaticity in the doubly reduced state of paaracyclophanetetraene,
- global aromaticity in the dication of [8]cycloparaphenylene
- through-space aromaticity in a stacked norcorrole dimer.
The underlying code is scheduled to be released within the next version of the TheoDORE wavefunction analysis package.